isolation of caffeine from tea
In this experiment we will isolate caffeine from tea. The reaction involves a homogenous mixture of an organic and aqueous layer.

Isolation Of Caffeine From Tea Ian Lee Pepito Academia Edu
02 M NaOH sodium hydroxide.
. Bring about 100 ml of water to boil in a 150 ml beaker. Ferric reducing antioxidant power FRAP of 010 mM Fe IIg. Ten commercial tea bags 2306 grams were used for the extraction.
Caffeine is present 1-4 in tea leaves while 1-2 in coffee seed. Place the tea bags in the boiling water and let them steep for 7 - 10 minutes with a watch glass. An average 30g of tea can contain 20-ll0 mg of caffeine thereby making tea a significant source of caffeine compared to other beverages.
Experiment 2 Isolation and Sublimation of Caffeine from Tea Leaves Reading Assignment Mohrig Chapter 10 extraction intro to Chapter 16 sublimation Extraction is the physical process by which a compound or mixture of compounds is transferred from one phase to another. Tea leaves are the rich source of caffeine which is a weak base. Additionally the compound is ideal for the isolation of caffeine from tea solution because it does not react with the solute.
Bring in two tea bags from home. In order to isolate caffeine from tea an extraction process. Aim of the experiment is to isolate and detect the active constituent caffeine present in tea dust.
In the case of Caffeine extraction from tea powder the solubility of caffeine in water is 22mgml at. Leaves are green in colour apex is blunt. Extract the Caffeine into a non-polar organic solvent.
Add about 4 g of sodium carbonate. Heat the water to boiling. The estimated value in tea leaves based on the assumption that 2 of the mass is caffeine.
An extraction is taking place each time coffee or tea is made. Boiling water 275 mL was used to extract caffeine from the solid leaves. Extraction unit TLC plate.
This happens when an organic. Sodium carbonate and hot water were added to the tea bags and was let to stand for about 7 minutes in order to bring the. Fortunately Caffeine is soluble in polar aprotic solvents whereas the Tannins are soluble in protic solvents due to hydrogen bonding.
This was the most efficient solvent because water causes tea to swell allowing for a caffeine solubility of 670 mgmL. In the first step we have to brew a very strong cup of tea. View Lab Report - Isolation of Caffeine from Tea from CHEM 243A at University Of Arizona.
Caffeine can be extracted from tea sweepings coffee roaster tea dust or tea. To determine the percent of caffeine per gram of instant tea. To perform a vacuum filtration 3.
To purify caffeine by recrystallization 5. Caffeine extraction from tea leaves involves an acidbase liquidliquid extraction Oneota 2003. Extract the Caffeine and Tannins into hot Water.
Here is an example of how to use solvent extraction to isolate and purify caffeine from tea. Caffeine can be obtained from different sources like Tea Camellia sinensis Family Theaceae coffee Coffea arabica Family- Rubiacea cacao Theobroma cacao Family Malvaceae cola Cola acuminata and Cola nitida Family- Malvaceae and others. Sodium carbonate was added to the teawater solution to ensure that caffeine.
2014 extracted the amount of caffeine from used tea leaves of black white green and red tea using dichloromethane. Furthermore dichloromethane is immiscible with water due to their differences in densities Williamson Masters 2010. To separate layers from a crude extract 4.
Download Solution Pharmacy Mobile App to Get All Uploaded Notes Model Question Papers Answer Papers Online Test and other GPAT Materials - httpsplay. Isolation of Caffeine. The ideal solvent in the extraction should have a low boiling point not react with the solute or other solvents not be toxic or highly flammable not miscible with.
Test the purity of your extracted caffeine using TLC. Thus we can carry-out the isolation of caffeine from tea leaves in the following steps. This data may be imprecise because during the extraction of caffeine emulsions may have occurred.
On the other hand. Thus these properties allow caffeine to be isolated from the aqueous mixture. The same principle may be used to extract other chemicals from natural sources.
To extract caffeine from tea 2. Extraction of Caffeine into water and then into ethyl acetate Place 120 mL of water in a 250 mL beaker and place it on a hot plate set to high. In this experiment a solid-liquid extraction method was used first to extract the caffeine from the tea leavestea bags to by dissolving sodium carbonate in hot water and creating an aqueous sodium carbonate solvent.
Because of the presence of Caffeine tea and coffee are gaining popularity as an addictive stimulant.

How To Extract Caffeine From Tea Classic Dcm Method Youtube

Solved Experiment 2 Isolation Of Caffeine From Tea Aims Chegg Com

Isolation And Characterization Of Caffeine From Waste Tea Semantic Scholar

Exp 2 Isolation And Sublimation Of Caffeine From Tea Leaves 4

Doc Isolation Of Caffeine From Tea Elizabeth Ping Academia Edu

Chm556 Experiment 1 Isolation Of Caffeine From A Tea Bag Pdf Solution Solvent
Lecture 3 A Extraction Of Caffeine From Tea

Extraction Isolation Of Caffeine From Tea Leaves Notes

Aldawaa Isolation Of Caffeine From Tea
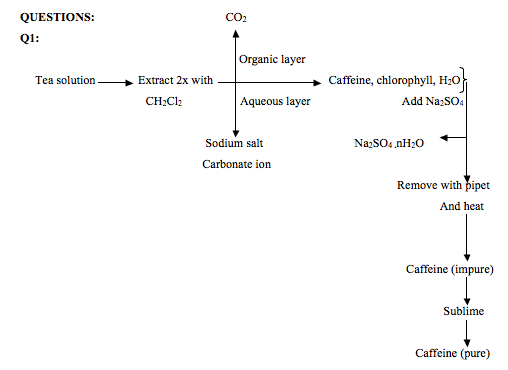
Caffeine Isolation From Tea Bags

Pdf Isolation And Characterization Of Caffeine From Waste Tea

One Part Of Chemistry Extraction Of Caffeine From Tea Leaves

Extraction And Isolation Of Caffeine From Tea Leaves English By Solution Pharmacy Youtube

Doc Isolation Of Caffeine From Tea Joshua Emmanuel Pagulong Academia Edu

Extraction Isolation Of Caffeine From Tea
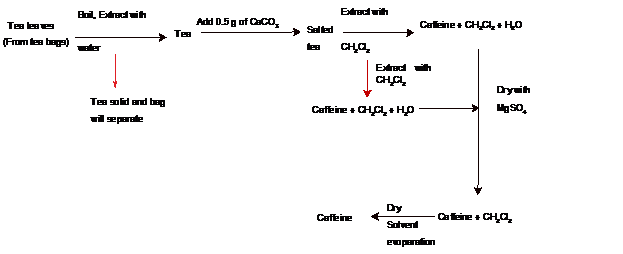
Theoutline Of A Separation Scheme For Isolating Caffeine From Tea Needs To Be Explained Concept Introduction In The Isolation Of Caffeine From Tea Leaves The Main Problem Is That The Caffeine Is
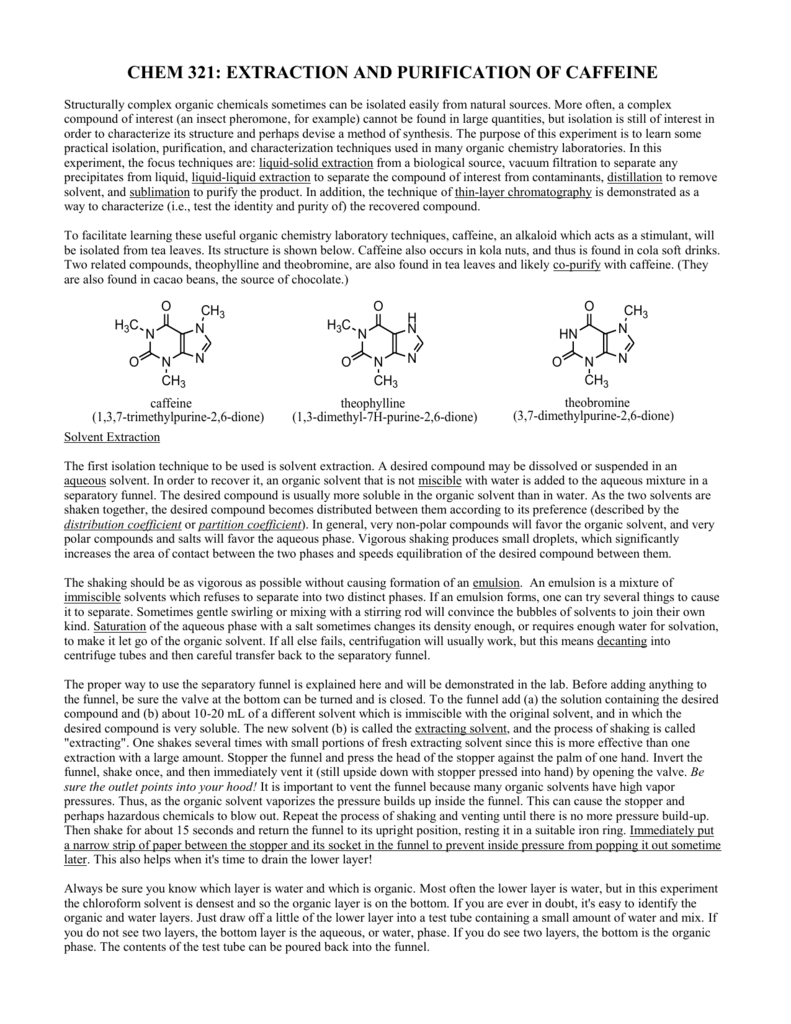
Experiment 2 Isolation Of Caffeine From Tea Leaves
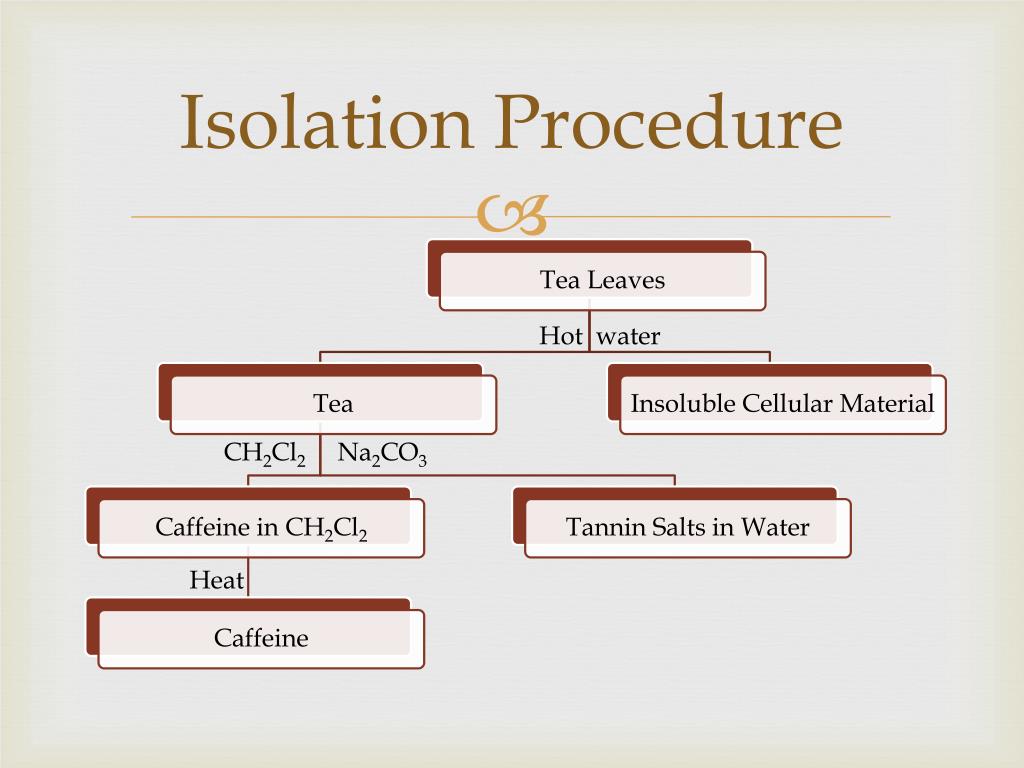
Ppt Isolation Of Caffeine From Tea Leaves Powerpoint Presentation Free Download Id 1607508

Lab Report Extraction Of Caffeine From Tea Lab Report Experiment Extraction Of Caffeine From Studocu
No comments for "isolation of caffeine from tea"
Post a Comment